Permeability Equations - Interstitial Velocity
It is defined as the velocity obtained by flow through the column between the particles. The free volume in the column available for flow is given by the left-hand equation below (equation 2.3):
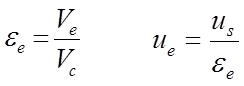
where Ve is the volume between the particles and Vc is the volume of the empty column. The interstitial solvent velocity ue relates to the superficial solvent velocity as given in the rhs of equation 2.3.
These definitions of solvent velocity come from chemical engineering. In chromatography, a third definition of solvent velocity is used mostly viz. the chromatographic solvent velocity. This velocity is easily determined as the quotient of the column length Lc and the hold-up time of an unretained solute t0 (equation 2.4).
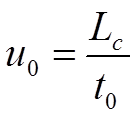 The subscript '0' in the velocity symbol u indicates that this value is based on t0. It is imperative now to realize that there are a few implicit approximations used.
The subscript '0' in the velocity symbol u indicates that this value is based on t0. It is imperative now to realize that there are a few implicit approximations used.
First t0 relates to the time a retained solute would have when it explores the inter- and interparticle space in the column with no retention. Since this is impossible, in practice t0 is estimated by a marker molecule in the sample that is supposed to be unretained.
Secondly, this time is measured at the point of detection of the HPLC system. This time is biased by the time the marker molecule spends in connection capillaries and other non-separating volumes between point of injection and point of detection. In practice, this error is not large, but one must be aware of these estimations. They become especially important when one intends to do physical-chemical measurements or chromatographic characterization of an HPLC column.
By physical parameters, u0 is described as in equation 2.5:
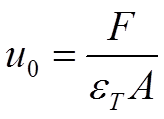
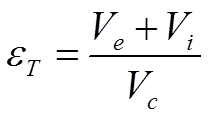 Here εT is the total porosity of the column given by equation 2.6 and Vi the available volume in the particle pores.
Here εT is the total porosity of the column given by equation 2.6 and Vi the available volume in the particle pores.
Analogous to equation 2.3 the chromatographic solvent velocity relates to superficial velocity by equation 2.7. This is important to realize since this is how the specific permeability given in equation 2.2. connects to chromatographic permeability in the rhs formula in equation 2.7.
 Here there may be a reason for some confusion since the specific permeability was called K0 and chromatographic permeability is called B0. The difference between K0 and B0 relates to the solvent velocity that is used to define it viz. superficial vs. chromatographic solvent velocity. One should be aware of this.
Here there may be a reason for some confusion since the specific permeability was called K0 and chromatographic permeability is called B0. The difference between K0 and B0 relates to the solvent velocity that is used to define it viz. superficial vs. chromatographic solvent velocity. One should be aware of this.
Next, we will look at how the porous structure of the particle bed relates to the specific resp. the chromatographic permeability.
